Figure 1
Two main morphotypes present in Viridiscus (PCM): (A) pores present, epicuticular granules typically reduced: the usual morph of V. viridissimus (Tennessee, dorsal view); (B) an aberrant specimen of V. viridissimus (Tennessee, dorsolateral view, the first median plate merged with the first paired segmental plate); (C) epicuticular granules dominant, pores present only in larval stage: V. viridianus (Alabama, dorsolateral view). Scale bars = 50 μm.
Figure 2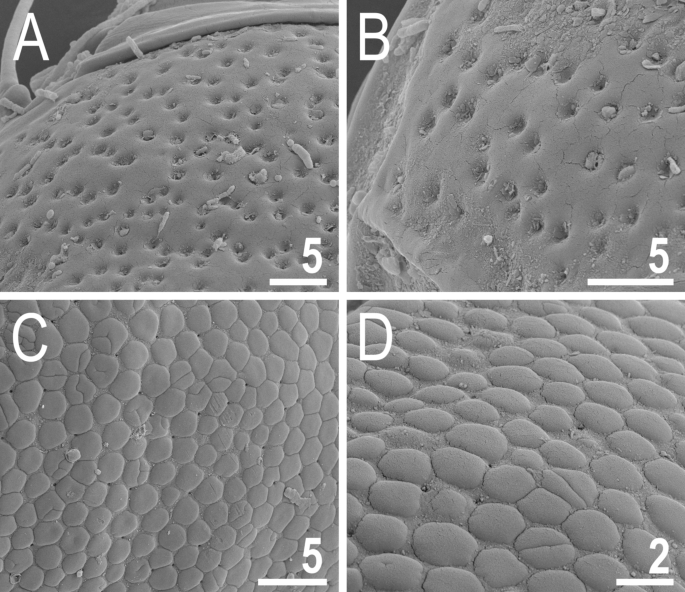
Two main morphotypes of dorsal plate sculpturing present in Viridiscus (SEM): pores dominant, only V. viridissimus (the population from Vietnam): (A) a fragment of the scapular plate; (B) close up of the posterior portion of the second paired segmental plate; epicuticular granules dominant, micropores visible only in SEM, all remaining Viridiscus species (the population of V. perviridis from Vietnam shown): (C) a fragment of the scapular plate; (D) close up of the central portion of the scapular plate. Scale bars in ÎĽm.
Viridiscus viridissimus exhibits considerable intra-specific variation in the morphology of dorsal armour17. In the typical morph (Fig. 3A), reliably recorded from the Holarctic and Oriental regions17, epicuticular granules are limited to the anterior portion of the second median and paired segmental plates, and to the third median plate. In contrast, the much less common morph of V. viridissimus (initially described as V. miraviridis Nelson et al., 202016), so far identified only among moss samples from Tennessee, exhibits epicuticular granules in all plate portions (Fig. 3B). Having the possibility to analyse abundant populations of V. perviridis and V. viridianus, we discovered atypical morphs in these species, too. In V. perviridis, the usually well-discernible epicuticular granules (Fig. 3C) can be poorly developed (and therefore blurred with endocuticle in PCM), especially in the scapular and caudal (terminal) plates (Fig. 3D). In V. viridianus, the reduction of epicuticular granules (Fig. 3E) can be even more pronounced to the extent that granules are absent, and only the intracuticular sponge layer is identifiable in PCM (Fig. 3F). These atypical morphs were not associated with a particular life stage or sex but appeared in large monospecific populations of the analysed Viridiscus spp. No atypical morphs were observed in the case of V. celatus sp. nov. (Fig. 3G,H), but the available sample size was significantly smaller in the case of the new species compared to the other analysed Viridiscus spp. As already stated in the diagnosis of the genus15, all known larvae of Viridiscus spp., irrespectively of the adult morphotype (type I or II), possess large pores beside granules (Fig. 4). We confirmed the presence of such pores in larvae of V. perviridis, V. viridianus, and V. viridissimus, and they have been also detected in V. clavispinosus24. Larvae of V. viridis s.s. (inhabiting the Hawaiian Archipelago) have never been found19,26.
Figure 3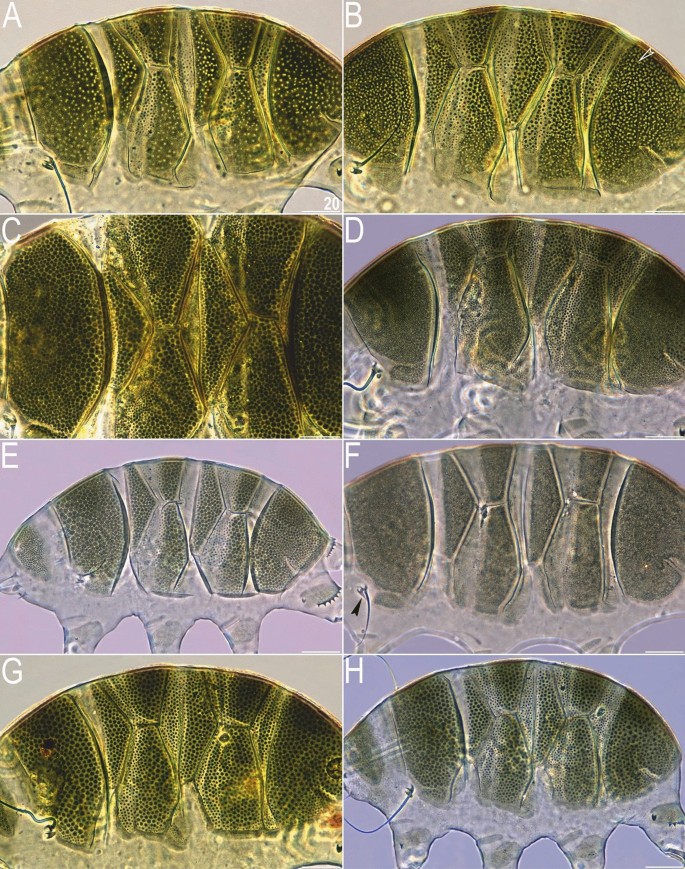
Intrageneric and intraspecific variability in Viridiscus (PCM): (A) V. viridissimus (Tennessee), the typical morph; (B) V. viridissimus (Tennessee), the atypical morph with well-developed epicuticular granules16; (C) V. perviridis (Madeira), the typical morph with well-developed epicuticular granules; (D) V. perviridis (Alabama), the atypical morph with poorly delineated epicuticular granules; (E) V. viridianus (Alabama), the typical morph with well-developed epicuticular granules; (F) V. viridianus (Alabama), the atypical morph (male) with epicuticular granules absent (arrowhead points out conoid primary clava); (G,H) V. celatus sp. nov. (Tennessee), the only morph observed. Scale bars = 20 μm.
Figure 4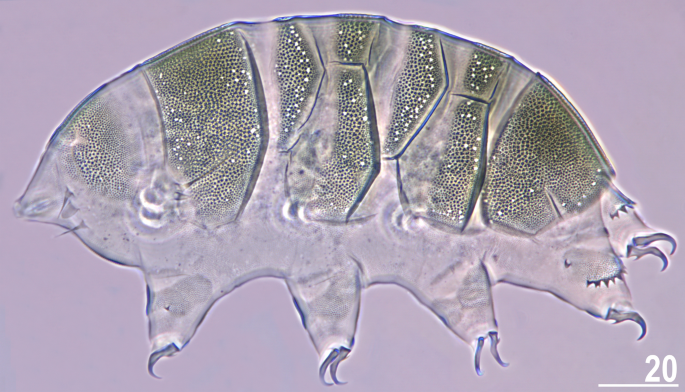
Larva of V. viridianus (PCM, dorsolateral view). Scale bar = 20 μm.
The phylogeny fully conformed with the morphological analyses, indicating the presence of four species (Fig. 5), relationships among which were as follows: (((V. perviridis (V. celatus sp. nov. (V. viridianus + V. viridissimus))). Viridiscus celatus sp. nov. was classified in17 as V. aff. viridianus. Importantly, species delimitations based on COI did not distinguish between V. perviridis and V. celatus sp. nov., which are clearly separated based on both ITS markers (Fig. 5) and morphology, pinpointing the questionable utility of the COI marker in tardigrade species delimitation. Given the abundant material and the proximity of the type locality in Auburn (Alabama) to the localities sampled in this study, we redescribe V. viridianus to provide a detailed insight into its intraspecific variability.
Figure 5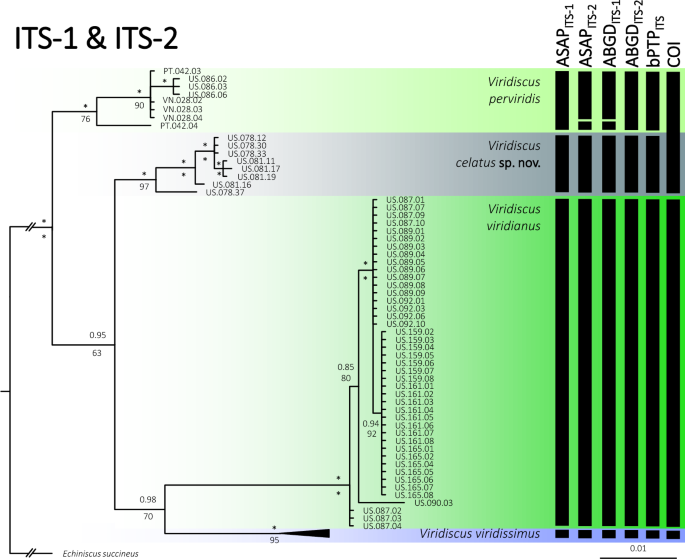
Phylogenetic relationships of the genus Viridiscus: Bayesian tree based on the concatenated ITS-1 + ITS-2 dataset (1058 bp); vertical bars denote different delineation methods used in the formulation of the primary molecular species hypotheses: ASAP, ABGD, and bPTP; (COI) refers to COI delimitation in all three methods). Asterisks indicate the maximal (1.00/100) posterior probability/bootstrap value. Echiniscus succineus was used as an outgroup. Scale bar represents substitutions per position.
Integrative redescription of Viridiscus viridianus (Pilato et al., 2007)
(Tables 1, 2, 3, 4, Figs. 6, 7, 8, 9, 10, 11, raw morphometry in Supplementary Material 1).
Table 1 Measurements (in µm) of selected morphological structures of adult females of Viridiscus viridianus mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Table 2 Measurements (in µm) of selected morphological structures of adult males of Viridiscus viridianus mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Table 3 Measurements (in µm) of selected morphological structures of juveniles of Viridiscus viridianus mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Table 4 Measurements (in µm) of selected morphological structures of larvae of Viridiscus viridianus mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Figure 6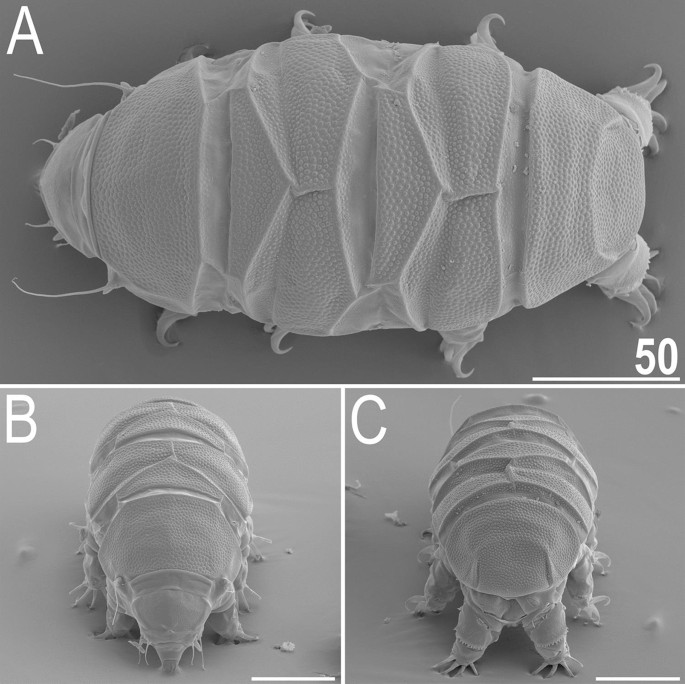
Habitus of V. viridianus (SEM): (A) dorsal view; (B) frontal view; (C) rear view. Scale bars = 50 μm.
Figure 7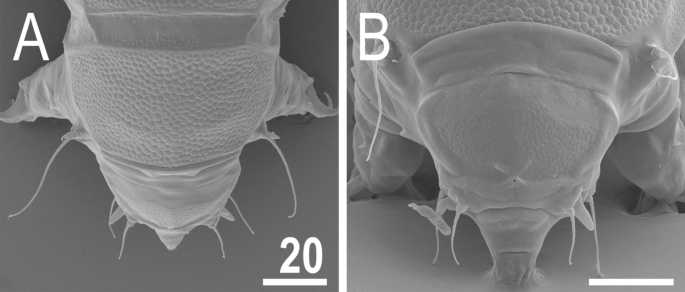
Anterior body portion of V. viridianus (SEM): (A) dorsal view; (B) head. Scale bars = 20 μm.
Figure 8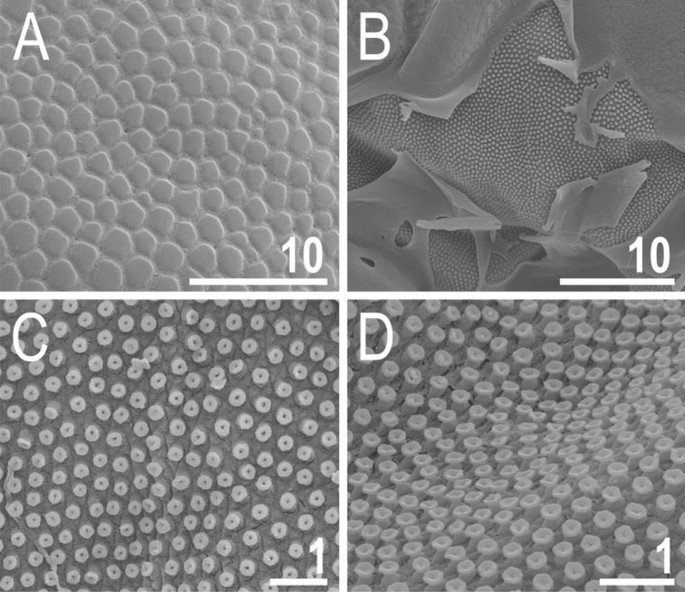
Cuticle of V. viridianus (SEM): (A) epicuticular granules and micropores; (B) lateral portion of disrupted epicuticle; (C,D) close up of intracuticular pillars. Scale bars in ÎĽm.
Figure 9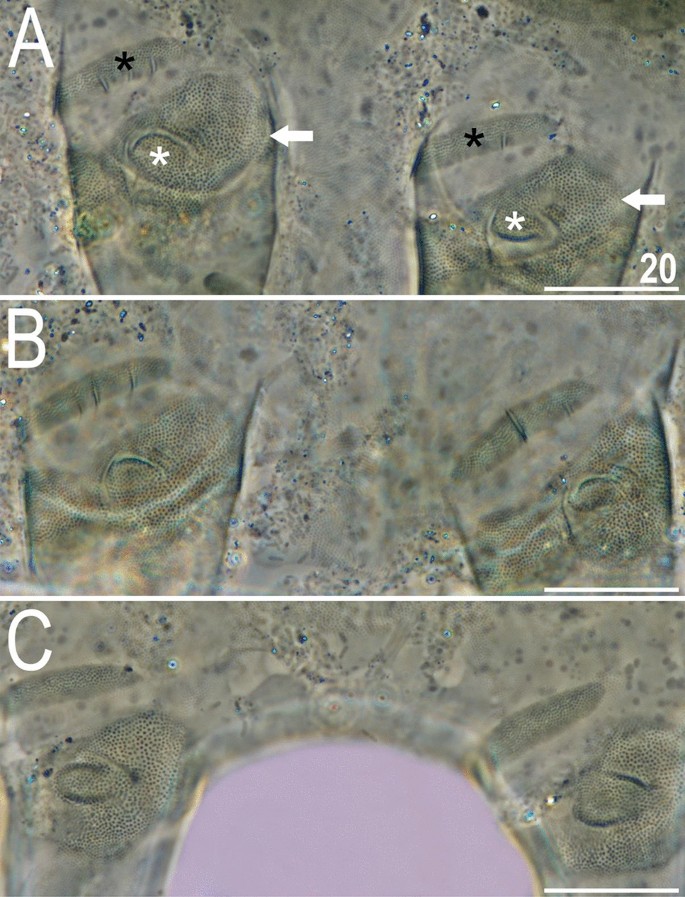
Leg morphology of V. viridianus (PCM). Arrows indicate pedal platelets in central leg portions, white asterisks indicate distinctly demarcated, central oval areas in pedal platelets, and black asterisks indicate pulvini in proximal leg portions. Scale bars = 20 μm.
Figure 10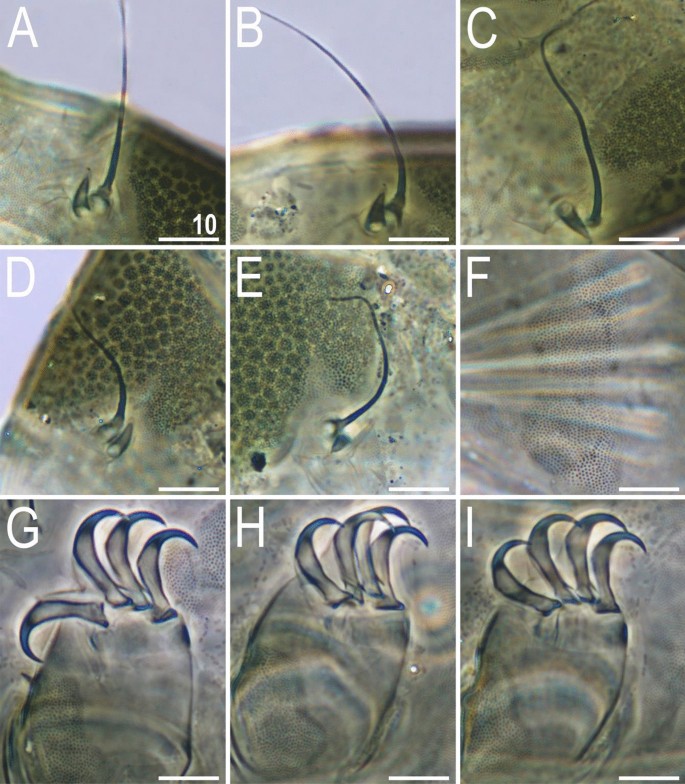
Morphological details of V. viridianus (PCM): (A–C) conoid primary clava; (D) dactyloid primary clava; (E) typical for most echiniscids, tubby Echiniscus-like primary clava; (F) subcephalic plates; (G) claws I; (H) claws II; (I) claws III. Scale bars = 10 μm.
Figure 11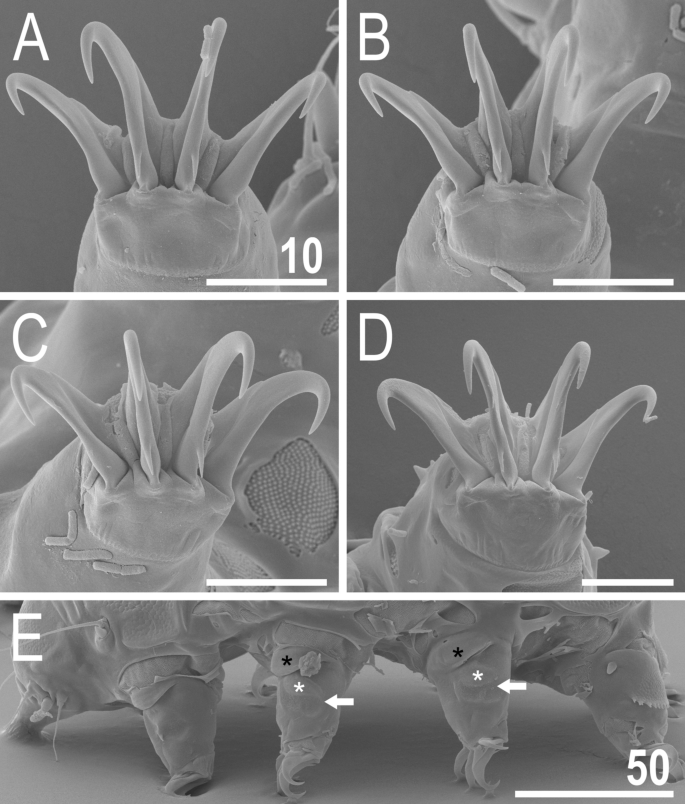
Leg structures of V. viridianus (SEM): (A) claws I; (B) claws II; (C) claws III; (D) claws IV; (E) leg morphology. Arrows indicate pedal platelets in central leg portions, white asterisks indicate distinctly demarcated, central oval areas in pedal platelets, and black asterisks indicate pulvini in proximal leg portions. Scale bars in ÎĽm.
New material examined. Populations from Alabama and Florida, 346 specimens in total were processed for PCM, SEM and DNA analyses (Table 5); additional few hundred specimens were frozen for future analyses.
Table 5 List of newly found populations used in analyses. Types of analyses: (PCM) imaging and morphometry in PCM, (SEM) imaging in SEM, (DNA) DNA sequencing. Number in each analysis indicates how many specimens were utilised in a given method (a adults, v exuvia, j juveniles, l larvae).
Type locality. North America, USA, Alabama, Auburn.
Additional localities. North America, USA, New Mexico23; the Azores, Ribeira Fria, Lages do Pico23,29,30; North America, USA, New Jersey28; Central America, the Lesser Antilles, Antigua28. Given the reported variability in the pattern of the dorsal armour, these additional localities should be verified.
Etymology. From Latin viridianus = greenish. An adjective in nominative singular.
Animals. Females (i.e., from the third instar onwards; measurements and statistics in Table 1). Body cavity with yellowish pigments (typical for most echiniscids), whereas dorsal and pedal cuticular plates light to dark green (Figs. 1C, 3E,F). Red eyes and yellow pigments present in live specimens, but dissolve after mounting in Hoyer’s medium, thus only green pigmentation persists. Body bulky (Fig. 6), with a poorly delimited cephalic region (Fig. 7). The cervical (neck) plate is well-developed, but sculptureless (Fig. 7). Weakly developed lateralmost, rectangular portions of the scapular plate with a weak sculpturing (Fig. 1C). Dorsal plate sculpturing ordinarily comprising polygonal epicuticular granules with scarce micropores, barely identifiable, even with SEM (Fig. 8A). Lateral and ventral endocuticle with intracuticular pillars, visible in PCM as minute dark dots, but identifiable in SEM only when the thin epicuticle is ruptured (Fig. 8B–D). Pillars larger and more sclerotised in proximal and central limb portions, forming longitudinal, narrow pulvini, and pedal platelets, respectively (Fig. 9). Some specimens exhibit a differently formed central pedal portion, more convex than the remainder of each platelet (Figs. 9, 11E). Areas of more sclerotised pillars always form a pair of merged subcephalic plates (Fig. 10F). Cirrus A short (7A, 10A–C), some have both clavae dactyloid, i.e., elongated, but without a pointed tip (Fig. 10D), and in some individuals both clavae are tubby, i.e., of a typical, Echiniscus-type shape (Fig. 10E). Importantly, numerous specimens showed a mixture of these shapes, that is the clava of one specimen differed in morphology from the other one on the same specimen. Claws massive and isonych (Figs. 10G–I, 11A–D).
Males (i.e., most probably from the third instar onwards; measurements and statistics in Table 2). No detectable sexual dimorphism besides the circular gonopore.
Juveniles (i.e., the second instar; measurements and statistics in Table 3). Smaller than adults, but qualitatively like them. Gonopore absent.
Larvae (i.e., the first instar; measurements and statistics in Table 4). Body size overlaps with juveniles. Anterior portions of paired segmental plates, and median plate 2 sculptureless. Large cuticular pores in the dorsal armour. No gonopore or anus.
Eggs. Up to five orange eggs per shed exuvia, but typically fewer (see31).
Remarks. Males were present in all examined populations of the species.
Description of Viridiscus
celatus sp. nov. Momeni, GÄ…siorek, Nelson & Michalczyk
(Tables 6, 7, Figs. 12, 13, 14, raw morphometry in Supplementary Material 2). ZooBank registration number: urn:lsid:zoobank.org:act:7E5416A6-E49F-46C2-8BA6-3D734B2A10A4.
Table 6 Measurements (in µm) of selected morphological structures of adult females of Viridiscus celatus sp. nov. mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Table 7 Measurements (in µm) of selected morphological structures of adult males and juveniles of Viridiscus celatus sp. nov. mounted in Hoyer’s medium. sp the proportion between the length of a given structure and the length of the scapular plate, ? unknown.Figure 12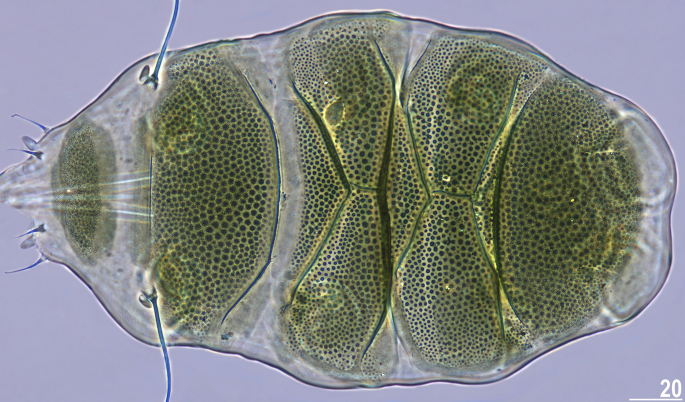
Holotype of V. celatus sp. nov. (PCM, female, dorsal view). Scale bar = 20 μm.
Figure 13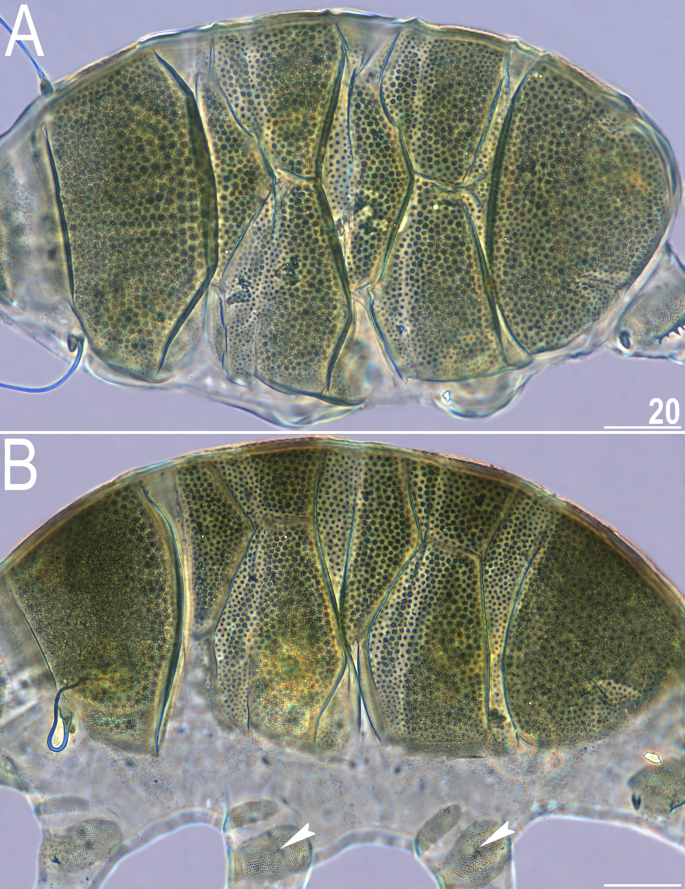
Type specimens of V. celatus sp. nov. (PCM): (A) allotype (male, dorsal view); (B) paratype (female, dorsolateral view). Arrowheads indicate rudimentary papillae on legs II–III. Scale bars = 20 μm.
Figure 14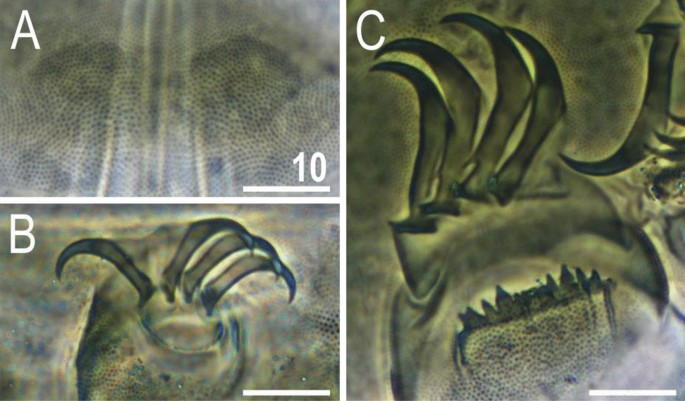
Morphological details of V. celatus sp. nov. (PCM): (A) subcephalic plates (holotype); (B) claws III (paratype, juvenile); (C) claws IV (holotype). Scale bars = 10 μm.
Material examined. Populations from Tennessee, 19 specimens in total processed for PCM and DNA analyses (Table 5).
Type locality. 36°18′N, 82°22′W, ca. 520 m asl: USA, Tennessee, Washington County, Johnson City. Grimmia sp. mosses from a concrete cap on a brick fence post.
Etymology. From Latin celatus = hidden, concealed. The name refers to the fact that the new species was not identified as new taxon for a long time, although the locality has been extensively sampled for tardigrades (16,27 referred to the species as V. perviridis based on the identification by Maucci32, and morphological characters, including cirrus A length). An adjective in nominative singular.
Type depositories: Type series: holotypic female (slide US.081.03), allotypic male (slide US.081.02), and nine paratypes (slides US.078.03 and US.081.03-4), are deposited at the Faculty of Biology, Jagiellonian University (KrakĂłw, Poland).
Animals. Females (i.e., from the third instar onwards; measurements and statistics in Table 6). Body medium-sized and bulky. Body cavity with yellowish pigments (typical for most echiniscids), whereas dorsal and pedal cuticular plates olive green (Figs. 1C, 3E,F). Red eyes and yellow pigments present in live specimens, but dissolve after mounting in Hoyer’s medium, thus only green pigmentation persists (Figs. 12, 13B). Except for cirrus A, with a tubby clava near the cirrophore (Fig. 12), other dorsal and lateral trunk appendages are absent. Cephalic appendages include internal and external peribuccal cirri with tubby cephalic papillae between them (Fig. 12). Dorsal plate sculpturing comprises large epicuticular granules (Fig. 12), which may be poorly developed in central plate portions (Fig. 13B). Sponge layer identifiable beneath granules. Granules appear more convex in anterior portions of paired segmental plates than in the remainder of the armour in PCM. Micropores not visible in PCM and their presence or absence remains to be confirmed in SEM.
All plates strongly sclerotised and with clear edges. The cephalic plate with a well-marked anterior chalice-shaped incision, the cervical plate and lateral sections of the body lack dense granulation and are covered with fine regular punctuation. The scapular plate contains three portions. Only the central part is visible in the dorsal view, and two small, weakly delineated, trapezoidal sections are present on the lateral portions of the body, with intracuticular pillars visible (Fig. 13B). The first median plate is triangular and unipartite, the second median plate is subdivided into two portions, and the anterior portion lacks the sponge layer. The third median plate is absent, but the area between the paired segmental plate II and the caudal plate is covered with large granules. Paired segmental plates I and II have two clearly delineated parts. Intersegmental plate is inserted between the posterolateral edge of the paired segmental plate I and anterior margin of paired segmental plate II. The caudal incisions are unsclerotised and weakly marked (Figs. 12, 13B).
Venter densely granulated in PCM (endocuticular pillars); a pair of subcephalic plates present (Fig. 14A). Gonopore hexapartite. Pulvini (= narrow proximal bands of intracuticular pillars) and pedal platelets (= broad central bands of pillars) are visible on all legs. Dentate collar with numerous irregular teeth (Fig. 14C). Sensory organs present on all legs: a tiny spine on leg I embedded at the edge of pedal platelet; hemispherical rudimentary papillae on legs II–III, embedded in the centre of pedal platelets (identifiable only when specimens are dorsolaterally oriented); and papilla IV on hind legs (Fig. 13B). Claws anisonych; primary spurs I–III tiny and thin, positioned slightly lower on branches compared to more massive spurs IV (Fig. 14B–C).
Males (i.e., most probably from the third instar onwards; measurements and statistics in Table 7). No sexual dimorphism observable in body size or qualitative traits (Fig. 13A). Gonopore circular.
Juveniles (i.e., the second instar; measurements and statistics in Table 7). Smaller than both females and males. Qualitatively like adults; gonopore absent.
Larvae. Not found.
Eggs. Not found.
Remarks. Found only in association with large populations of V. viridissimus17.
Differential diagnosis. The new species from Tennessee is differentiated from all Viridiscus spp. based on the presence of plesiomorphic papillae on legs II–III. These structures are, however, barely identifiable in specimens oriented dorsoventrally, hence we enumerate other criteria making V. celatus sp. nov. distinct from:
Viridiscus clavispinosus, by the relative length of cirrus A (34–49% vs
Viridiscus perviridis, veritably reported from the Holarctic and Oriental regions17, by the length of cirrus A (34–49% vs typically  ≫ 50% of the body length, see the subsection below that addresses this character), the weakly developed caudal incisions (strongly sclerotised and well-marked in all syntypes of V. perviridis), and the body colour (light to olive green vs usually dark green to almost black in V. perviridis, also in mounted specimens);
Viridiscus viridianus, reliably reported only from the USA, by the relative length of cirrus A (34–49% vs
Viridiscus viridis, reliably reported only from the Hawaiian Archipelago19,26, by the relative length of cirrus A (34–49% vs V. viridis has noticeably fewer epicuticular granules on all plates, see fig. 1 in26);
Viridiscus viridissimus, with a likely wide distribution in the Holarctic, Oriental, and Neotropical regions17,33, by the absence of pores in dorsal armour, and a better developed sponge layer of cuticle.
Source link : https://www.nature.com/articles/s41598-023-40609-4
Author :
Publish date : 2023-09-28 03:00:00
Copyright for syndicated content belongs to the linked Source.